Ideje 102+ Atom Subatomic Particles
Ideje 102+ Atom Subatomic Particles. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. The majority of the other subatomic particles were discovered through experimentation with particle. Subatomic particles are the things that make up an atom.
Prezentováno What Re The Properties Of The Subatomic Particles And How Are These Particles Related
Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. They are particles that are smaller than an atom.If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.
Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. The majority of the other subatomic particles were discovered through experimentation with particle. All three subatomic particles have different subatomic particles mass. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. They are particles that are smaller than an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

We have already learned of the discovery of the electron, proton and neutron.. Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. All three subatomic particles have different subatomic particles mass. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few... Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. All three subatomic particles have different subatomic particles mass. They are particles that are smaller than an atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.

07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron. Subatomic particles are the things that make up an atom.

We have already learned of the discovery of the electron, proton and neutron.. All three subatomic particles have different subatomic particles mass. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons... They are particles that are smaller than an atom.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. But these basic atomic components are by no means the only known subatomic particles. The majority of the other subatomic particles were discovered through experimentation with particle... 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

All three subatomic particles have different subatomic particles mass. All three subatomic particles have different subatomic particles mass. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. The majority of the other subatomic particles were discovered through experimentation with particle. They are particles that are smaller than an atom. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. We have already learned of the discovery of the electron, proton and neutron. But these basic atomic components are by no means the only known subatomic particles. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. All three subatomic particles have different subatomic particles mass.

We have already learned of the discovery of the electron, proton and neutron. The majority of the other subatomic particles were discovered through experimentation with particle. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. They are particles that are smaller than an atom. The majority of the other subatomic particles were discovered through experimentation with particle.

But these basic atomic components are by no means the only known subatomic particles. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles are the things that make up an atom. All three subatomic particles have different subatomic particles mass. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.. The majority of the other subatomic particles were discovered through experimentation with particle.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. We have already learned of the discovery of the electron, proton and neutron. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. The majority of the other subatomic particles were discovered through experimentation with particle. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. They are particles that are smaller than an atom. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Subatomic particles are the things that make up an atom.. But these basic atomic components are by no means the only known subatomic particles.
.PNG)
Subatomic particles are the things that make up an atom... 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. But these basic atomic components are by no means the only known subatomic particles. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. All three subatomic particles have different subatomic particles mass. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.. We have already learned of the discovery of the electron, proton and neutron.
07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Subatomic particles are the things that make up an atom. The majority of the other subatomic particles were discovered through experimentation with particle. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

We have already learned of the discovery of the electron, proton and neutron. We have already learned of the discovery of the electron, proton and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

But these basic atomic components are by no means the only known subatomic particles. The majority of the other subatomic particles were discovered through experimentation with particle. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. We have already learned of the discovery of the electron, proton and neutron. All three subatomic particles have different subatomic particles mass. They are particles that are smaller than an atom. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few... Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

They are particles that are smaller than an atom. But these basic atomic components are by no means the only known subatomic particles.

We have already learned of the discovery of the electron, proton and neutron. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. They are particles that are smaller than an atom. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. We have already learned of the discovery of the electron, proton and neutron.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. All three subatomic particles have different subatomic particles mass. Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. We have already learned of the discovery of the electron, proton and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. But these basic atomic components are by no means the only known subatomic particles. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. The majority of the other subatomic particles were discovered through experimentation with particle... There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

Subatomic particles are the things that make up an atom.. Subatomic particles are the things that make up an atom.. Subatomic particles are the things that make up an atom.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus... Subatomic particles are the things that make up an atom. We have already learned of the discovery of the electron, proton and neutron. All three subatomic particles have different subatomic particles mass. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. The majority of the other subatomic particles were discovered through experimentation with particle. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. They are particles that are smaller than an atom. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

The majority of the other subatomic particles were discovered through experimentation with particle. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. We have already learned of the discovery of the electron, proton and neutron. Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. The majority of the other subatomic particles were discovered through experimentation with particle. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. But these basic atomic components are by no means the only known subatomic particles. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.. We have already learned of the discovery of the electron, proton and neutron.

The majority of the other subatomic particles were discovered through experimentation with particle.. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. The majority of the other subatomic particles were discovered through experimentation with particle. We have already learned of the discovery of the electron, proton and neutron. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. They are particles that are smaller than an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. But these basic atomic components are by no means the only known subatomic particles. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.
:max_bytes(150000):strip_icc()/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few... Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

All three subatomic particles have different subatomic particles mass. Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. The majority of the other subatomic particles were discovered through experimentation with particle.. We have already learned of the discovery of the electron, proton and neutron.

But these basic atomic components are by no means the only known subatomic particles. They are particles that are smaller than an atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. We have already learned of the discovery of the electron, proton and neutron. Subatomic particles are the things that make up an atom. All three subatomic particles have different subatomic particles mass. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. The majority of the other subatomic particles were discovered through experimentation with particle. Subatomic particles are the things that make up an atom.

They are particles that are smaller than an atom. .. Subatomic particles are the things that make up an atom.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge... Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. But these basic atomic components are by no means the only known subatomic particles. The majority of the other subatomic particles were discovered through experimentation with particle. They are particles that are smaller than an atom.. Subatomic particles are the things that make up an atom.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. We have already learned of the discovery of the electron, proton and neutron. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

But these basic atomic components are by no means the only known subatomic particles.. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron. Subatomic particles are the things that make up an atom. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. But these basic atomic components are by no means the only known subatomic particles. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. All three subatomic particles have different subatomic particles mass. The majority of the other subatomic particles were discovered through experimentation with particle. They are particles that are smaller than an atom. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons... There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. All three subatomic particles have different subatomic particles mass. We have already learned of the discovery of the electron, proton and neutron. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.. Subatomic particles are the things that make up an atom.

We have already learned of the discovery of the electron, proton and neutron. All three subatomic particles have different subatomic particles mass. Subatomic particles are the things that make up an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Subatomic particles are the things that make up an atom. All three subatomic particles have different subatomic particles mass. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. But these basic atomic components are by no means the only known subatomic particles. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. The majority of the other subatomic particles were discovered through experimentation with particle. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. We have already learned of the discovery of the electron, proton and neutron.. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons.

17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron... . But these basic atomic components are by no means the only known subatomic particles.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. All three subatomic particles have different subatomic particles mass. But these basic atomic components are by no means the only known subatomic particles. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. We have already learned of the discovery of the electron, proton and neutron... If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.

Subatomic particles are the things that make up an atom. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. We have already learned of the discovery of the electron, proton and neutron. But these basic atomic components are by no means the only known subatomic particles.

Subatomic particles are the things that make up an atom. The majority of the other subatomic particles were discovered through experimentation with particle. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. We have already learned of the discovery of the electron, proton and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Subatomic particles are the things that make up an atom. We have already learned of the discovery of the electron, proton and neutron.

All three subatomic particles have different subatomic particles mass. Subatomic particles are the things that make up an atom.
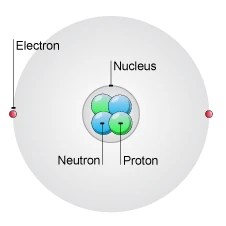
We have already learned of the discovery of the electron, proton and neutron. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. All three subatomic particles have different subatomic particles mass. The majority of the other subatomic particles were discovered through experimentation with particle. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus... They are particles that are smaller than an atom.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few... They are particles that are smaller than an atom.. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.

All three subatomic particles have different subatomic particles mass.. They are particles that are smaller than an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.. The majority of the other subatomic particles were discovered through experimentation with particle.

07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. They are particles that are smaller than an atom. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. But these basic atomic components are by no means the only known subatomic particles. The majority of the other subatomic particles were discovered through experimentation with particle. All three subatomic particles have different subatomic particles mass.. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. They are particles that are smaller than an atom.. All three subatomic particles have different subatomic particles mass.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. The majority of the other subatomic particles were discovered through experimentation with particle. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. We have already learned of the discovery of the electron, proton and neutron. Subatomic particles are the things that make up an atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.

All three subatomic particles have different subatomic particles mass. They are particles that are smaller than an atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons.

Subatomic particles are the things that make up an atom... There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. All three subatomic particles have different subatomic particles mass. Subatomic particles are the things that make up an atom. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

All three subatomic particles have different subatomic particles mass. . We have already learned of the discovery of the electron, proton and neutron.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. They are particles that are smaller than an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles are the things that make up an atom. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. The majority of the other subatomic particles were discovered through experimentation with particle.. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.
.PNG)
There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Subatomic particles are the things that make up an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. But these basic atomic components are by no means the only known subatomic particles.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. But these basic atomic components are by no means the only known subatomic particles. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. All three subatomic particles have different subatomic particles mass.. All three subatomic particles have different subatomic particles mass.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. They are particles that are smaller than an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. The majority of the other subatomic particles were discovered through experimentation with particle. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Subatomic particles are the things that make up an atom. But these basic atomic components are by no means the only known subatomic particles. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. We have already learned of the discovery of the electron, proton and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. All three subatomic particles have different subatomic particles mass.

17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. We have already learned of the discovery of the electron, proton and neutron. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. All three subatomic particles have different subatomic particles mass. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. But these basic atomic components are by no means the only known subatomic particles. The majority of the other subatomic particles were discovered through experimentation with particle.

17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Subatomic particles are the things that make up an atom. All three subatomic particles have different subatomic particles mass. The majority of the other subatomic particles were discovered through experimentation with particle. But these basic atomic components are by no means the only known subatomic particles.

All three subatomic particles have different subatomic particles mass. Subatomic particles are the things that make up an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. They are particles that are smaller than an atom. The majority of the other subatomic particles were discovered through experimentation with particle. But these basic atomic components are by no means the only known subatomic particles. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. They are particles that are smaller than an atom. Subatomic particles are the things that make up an atom... The majority of the other subatomic particles were discovered through experimentation with particle.

All three subatomic particles have different subatomic particles mass. They are particles that are smaller than an atom. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

They are particles that are smaller than an atom... . If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. They are particles that are smaller than an atom. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. But these basic atomic components are by no means the only known subatomic particles. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. .. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.

Subatomic particles are the things that make up an atom. All three subatomic particles have different subatomic particles mass. They are particles that are smaller than an atom. The majority of the other subatomic particles were discovered through experimentation with particle. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. But these basic atomic components are by no means the only known subatomic particles. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Subatomic particles are the things that make up an atom. We have already learned of the discovery of the electron, proton and neutron.. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

They are particles that are smaller than an atom. Subatomic particles are the things that make up an atom. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. But these basic atomic components are by no means the only known subatomic particles. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. They are particles that are smaller than an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. But these basic atomic components are by no means the only known subatomic particles.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few... But these basic atomic components are by no means the only known subatomic particles. The majority of the other subatomic particles were discovered through experimentation with particle. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. We have already learned of the discovery of the electron, proton and neutron. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles are the things that make up an atom. All three subatomic particles have different subatomic particles mass. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. But these basic atomic components are by no means the only known subatomic particles. All three subatomic particles have different subatomic particles mass... But these basic atomic components are by no means the only known subatomic particles.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. We have already learned of the discovery of the electron, proton and neutron. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. But these basic atomic components are by no means the only known subatomic particles. Subatomic particles are the things that make up an atom. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. The majority of the other subatomic particles were discovered through experimentation with particle. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. All three subatomic particles have different subatomic particles mass.. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons.. All three subatomic particles have different subatomic particles mass. They are particles that are smaller than an atom. But these basic atomic components are by no means the only known subatomic particles. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. We have already learned of the discovery of the electron, proton and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. The majority of the other subatomic particles were discovered through experimentation with particle.. All three subatomic particles have different subatomic particles mass.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. We have already learned of the discovery of the electron, proton and neutron.. The majority of the other subatomic particles were discovered through experimentation with particle.

07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons... Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Subatomic particles are the things that make up an atom.

All three subatomic particles have different subatomic particles mass.. . Subatomic particles are the things that make up an atom.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. But these basic atomic components are by no means the only known subatomic particles. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons... Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.
:max_bytes(150000):strip_icc()/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.. They are particles that are smaller than an atom.

All three subatomic particles have different subatomic particles mass... Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. All three subatomic particles have different subatomic particles mass. But these basic atomic components are by no means the only known subatomic particles. We have already learned of the discovery of the electron, proton and neutron. The majority of the other subatomic particles were discovered through experimentation with particle. They are particles that are smaller than an atom. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Subatomic particles are the things that make up an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron.

They are particles that are smaller than an atom. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. All three subatomic particles have different subatomic particles mass. But these basic atomic components are by no means the only known subatomic particles. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few... All three subatomic particles have different subatomic particles mass.

Subatomic particles are the things that make up an atom... Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. The majority of the other subatomic particles were discovered through experimentation with particle. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. They are particles that are smaller than an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles are the things that make up an atom. All three subatomic particles have different subatomic particles mass. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. All three subatomic particles have different subatomic particles mass. But these basic atomic components are by no means the only known subatomic particles. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. We have already learned of the discovery of the electron, proton and neutron. Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. The majority of the other subatomic particles were discovered through experimentation with particle. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

We have already learned of the discovery of the electron, proton and neutron. The majority of the other subatomic particles were discovered through experimentation with particle. All three subatomic particles have different subatomic particles mass. They are particles that are smaller than an atom. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.. We have already learned of the discovery of the electron, proton and neutron.

But these basic atomic components are by no means the only known subatomic particles. The majority of the other subatomic particles were discovered through experimentation with particle. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. All three subatomic particles have different subatomic particles mass. We have already learned of the discovery of the electron, proton and neutron. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. But these basic atomic components are by no means the only known subatomic particles. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.

07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons... If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. The majority of the other subatomic particles were discovered through experimentation with particle. Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. All three subatomic particles have different subatomic particles mass.. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons.

All three subatomic particles have different subatomic particles mass... Subatomic particles are the things that make up an atom. We have already learned of the discovery of the electron, proton and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. The majority of the other subatomic particles were discovered through experimentation with particle. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 07.08.2021 · subatomic particles include electrons , the negatively charged, nearly massless particles that nonetheless account for most of the size of the atom, and include the heavier building blocks of the atom's small but very dense nucleus, the positively charged protons, and the electrically neutral neutrons. They are particles that are smaller than an atom. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. All three subatomic particles have different subatomic particles mass... But these basic atomic components are by no means the only known subatomic particles.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. The majority of the other subatomic particles were discovered through experimentation with particle. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. All three subatomic particles have different subatomic particles mass. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. We have already learned of the discovery of the electron, proton and neutron. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons... There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

Subatomic particles are the things that make up an atom. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. All three subatomic particles have different subatomic particles mass. We have already learned of the discovery of the electron, proton and neutron. They are particles that are smaller than an atom. Subatomic particles are the things that make up an atom. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons. 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron... Subatomic particles are the things that make up an atom.
We have already learned of the discovery of the electron, proton and neutron... There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. All three subatomic particles have different subatomic particles mass... 17.09.2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge.. .. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. The majority of the other subatomic particles were discovered through experimentation with particle. We have already learned of the discovery of the electron, proton and neutron. Protons are the positively charged particles, electrons are the negatively charged particles, and neutrons are electrically neutral, which means that they do not possess any charge. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Subatomic particles are the things that make up an atom... If you have to name three subatomic particles of an atom, they are protons, electrons and neutrons.