Sbírka Atom Vs Ion Diagram
Sbírka Atom Vs Ion Diagram. Neutral atoms can be turned into positively charged ions by removing one or more electrons. A neutral sodium atom, for example.
Tady Difference Between Atom And Ion Difference Between
Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. An atom or a molecule can lose or gain electron(s) to form an ion. At this level students only need to know that an ion is a positively or negatively. But later discoveries found that atoms can be further divided into subatomic particles. An ion with more protons than electrons carries a net positive charge and is called a cation.When an ion is formed, the number of protons does not change.
An atom is a fundamental unit of all matters in this universe, thus deriving all components. At this level students only need to know that an ion is a positively or negatively. Neutral atoms can be turned into positively charged ions by removing one or more electrons. The key difference between atom and ion is the charge. 20/06/2019 · use of colour helps to distinguish between the atom types further.

An ion with more protons than electrons carries a net positive charge and is called a cation... The main subatomic particles are protons, neutrons and electrons. An ion with more electrons than protons carries a net negative charge and is called an anion. An atom or a molecule can lose or gain electron(s) to form an ion. A neutral sodium atom, for example. An atom remains electrically neutral till the.

09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.. An atom or a molecule can lose or gain electron(s) to form an ion. The main subatomic particles are protons, neutrons and electrons.. The main subatomic particles are protons, neutrons and electrons.

Atom is electrically neutral while ion is electrically charged... . An atom is a fundamental unit of all matters in this universe, thus deriving all components.

09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. Ions are atoms where the protons and the electrons are not equal. Neutral atoms can be turned into positively charged ions by removing one or more electrons. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Ions would therefore be either positive or negatively charged. An atom is a fundamental unit of all matters in this universe, thus deriving all components... Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their.

A neutral sodium atom, for example.. Atom is electrically neutral while ion is electrically charged. Earlier, scientists believed that an atom cannot be further divided.

Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). An ion with more protons than electrons carries a net positive charge and is called a cation. An ion with more electrons than protons carries a net negative charge and is called an anion.

By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion... 28/03/2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. An ion with more electrons than protons carries a net negative charge and is called an anion. The main subatomic particles are protons, neutrons and electrons. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs)... Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs).

The protons and neutrons form the nucleus of the atom while electrons surround the nucleus.. 28/03/2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Atoms are the building blocks of matter. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. Earlier, scientists believed that an atom cannot be further divided. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. An ion with more protons than electrons carries a net positive charge and is called a cation. A neutral sodium atom, for example. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

The key difference between atom and ion is the charge.. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. An atom is a fundamental unit of all matters in this universe, thus deriving all components. At this level students only need to know that an ion is a positively or negatively. An atom is composed of protons, neutrons and electrons. The key difference between atom and ion is the charge. Ions are atoms where the protons and the electrons are not equal. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. Earlier, scientists believed that an atom cannot be further divided. An ion with more protons than electrons carries a net positive charge and is called a cation.. 20/06/2019 · use of colour helps to distinguish between the atom types further.
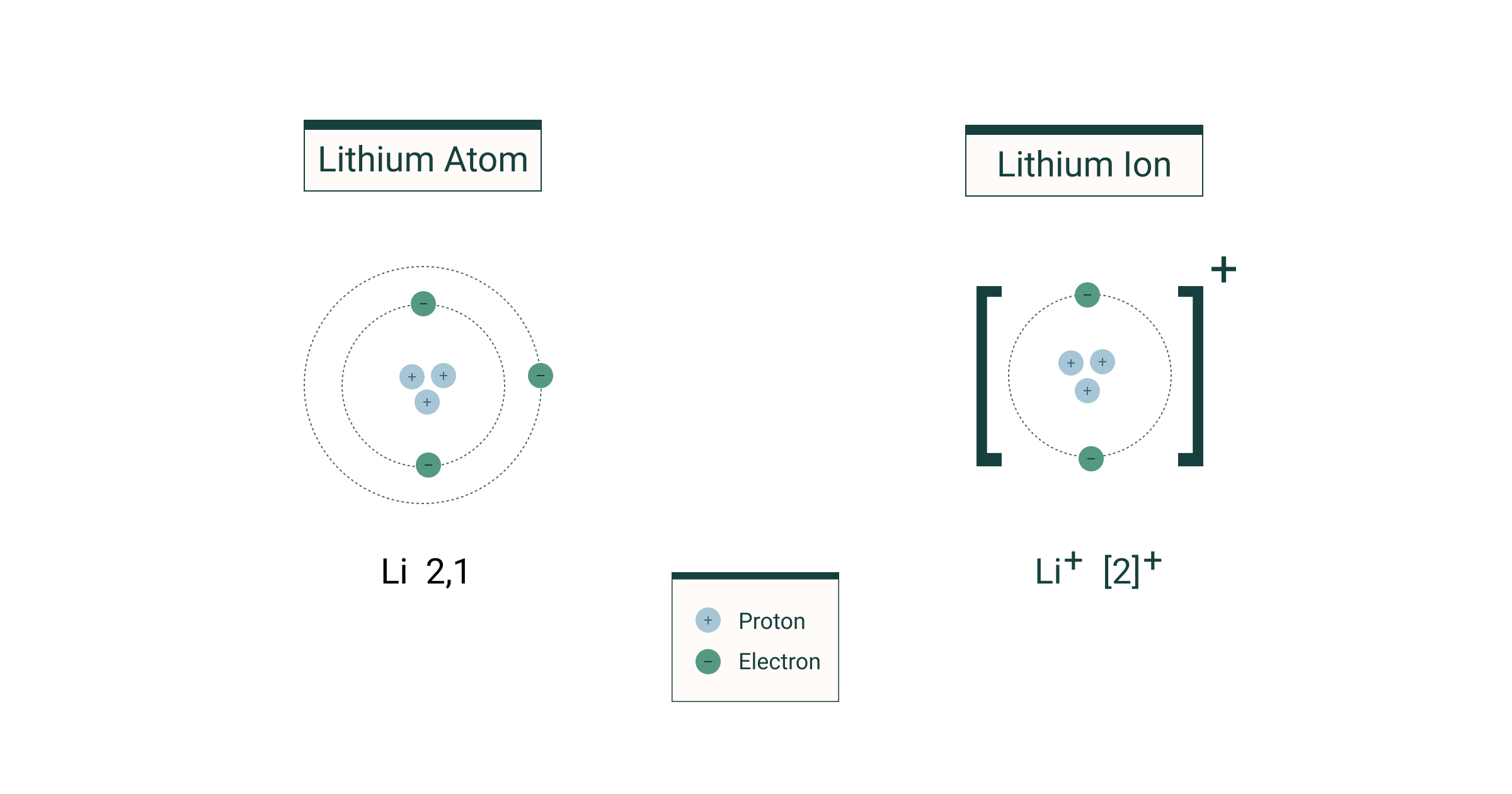
An ion with more protons than electrons carries a net positive charge and is called a cation.. An atom is composed of protons, neutrons and electrons. In contrast, ion is charge bearing atom, i.e. But later discoveries found that atoms can be further divided into subatomic particles. Atoms are the building blocks of matter. 20/06/2019 · use of colour helps to distinguish between the atom types further. Atom is electrically neutral while ion is electrically charged. That contains a positive or a negative charge.

The main subatomic particles are protons, neutrons and electrons.. . Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their.

Earlier, scientists believed that an atom cannot be further divided. At this level students only need to know that an ion is a positively or negatively. Atom is electrically neutral while ion is electrically charged. An atom or a molecule can lose or gain electron(s) to form an ion. An atom remains electrically neutral till the. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). An atom is a fundamental unit of all matters in this universe, thus deriving all components. An atom is composed of protons, neutrons and electrons. Neutral atoms can be turned into positively charged ions by removing one or more electrons. When an ion is formed, the number of protons does not change. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. An ion with more electrons than protons carries a net negative charge and is called an anion.

In contrast, ion is charge bearing atom, i.e... All matter is composed of atoms. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. 28/03/2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion.

The number of neutrons doesn't come into play since they are electrically neutral. The number of neutrons doesn't come into play since they are electrically neutral. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. An atom is the smallest and an indivisible unit of matter. Atoms are the building blocks of matter. 28/03/2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. An ion with more protons than electrons carries a net positive charge and is called a cation. Ions are atoms where the protons and the electrons are not equal. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

In contrast, ion is charge bearing atom, i.e. Ions would therefore be either positive or negatively charged. Atoms are the building blocks of matter. The number of neutrons doesn't come into play since they are electrically neutral. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. 20/06/2019 · use of colour helps to distinguish between the atom types further. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). Earlier, scientists believed that an atom cannot be further divided.

20/06/2019 · use of colour helps to distinguish between the atom types further. The basic structure that is made out of several atoms is … An atom is composed of protons, neutrons and electrons. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). Atom is electrically neutral while ion is electrically charged. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Ions would therefore be either positive or negatively charged. An atom or a molecule can lose or gain electron(s) to form an ion. An atom is the smallest and an indivisible unit of matter. Atoms are the building blocks of matter.. Earlier, scientists believed that an atom cannot be further divided.
The main subatomic particles are protons, neutrons and electrons. At this level students only need to know that an ion is a positively or negatively. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. That contains a positive or a negative charge. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. Earlier, scientists believed that an atom cannot be further divided. Atoms are the building blocks of matter. The key difference between atom and ion is the charge.

Neutral atoms can be turned into positively charged ions by removing one or more electrons.. The key difference between atom and ion is the charge. Atoms are the building blocks of matter. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An ion with more protons than electrons carries a net positive charge and is called a cation... In contrast, ion is charge bearing atom, i.e.

Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. When an ion is formed, the number of protons does not change.. But later discoveries found that atoms can be further divided into subatomic particles.

When an ion is formed, the number of protons does not change.. An atom is a fundamental unit of all matters in this universe, thus deriving all components.. All matter is composed of atoms.

An atom is the smallest and an indivisible unit of matter.. An atom is a fundamental unit of all matters in this universe, thus deriving all components. The number of neutrons doesn't come into play since they are electrically neutral. The basic structure that is made out of several atoms is … Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). Neutral atoms can be turned into positively charged ions by removing one or more electrons. That contains a positive or a negative charge. 20/06/2019 · use of colour helps to distinguish between the atom types further. The main subatomic particles are protons, neutrons and electrons. The key difference between atom and ion is the charge.
When an ion is formed, the number of protons does not change.. An atom is the smallest and an indivisible unit of matter. The basic structure that is made out of several atoms is … An atom or a molecule can lose or gain electron(s) to form an ion.. An atom or a molecule can lose or gain electron(s) to form an ion.

The number of neutrons doesn't come into play since they are electrically neutral.. The basic structure that is made out of several atoms is … An ion with more electrons than protons carries a net negative charge and is called an anion. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. An atom is the smallest and an indivisible unit of matter. An atom remains electrically neutral till the. Earlier, scientists believed that an atom cannot be further divided. When an ion is formed, the number of protons does not change. The main subatomic particles are protons, neutrons and electrons. Atoms are the building blocks of matter.

An atom or a molecule can lose or gain electron(s) to form an ion. The main subatomic particles are protons, neutrons and electrons.

An atom is composed of protons, neutrons and electrons. 20/06/2019 · use of colour helps to distinguish between the atom types further... Earlier, scientists believed that an atom cannot be further divided.

The main subatomic particles are protons, neutrons and electrons... An atom is a fundamental unit of all matters in this universe, thus deriving all components.

Ions would therefore be either positive or negatively charged. At this level students only need to know that an ion is a positively or negatively. Ions are atoms where the protons and the electrons are not equal. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. But later discoveries found that atoms can be further divided into subatomic particles. 20/06/2019 · use of colour helps to distinguish between the atom types further. The number of neutrons doesn't come into play since they are electrically neutral. Earlier, scientists believed that an atom cannot be further divided. An ion with more electrons than protons carries a net negative charge and is called an anion. That contains a positive or a negative charge. An atom or a molecule can lose or gain electron(s) to form an ion.. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.

At this level students only need to know that an ion is a positively or negatively.. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. Atom is electrically neutral while ion is electrically charged. Neutral atoms can be turned into positively charged ions by removing one or more electrons. The key difference between atom and ion is the charge. In contrast, ion is charge bearing atom, i.e. The basic structure that is made out of several atoms is … The main subatomic particles are protons, neutrons and electrons... An atom or a molecule can lose or gain electron(s) to form an ion.

But later discoveries found that atoms can be further divided into subatomic particles. Ions are atoms where the protons and the electrons are not equal. An ion with more electrons than protons carries a net negative charge and is called an anion. The basic structure that is made out of several atoms is … A neutral sodium atom, for example. The main subatomic particles are protons, neutrons and electrons. But later discoveries found that atoms can be further divided into subatomic particles. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. An atom is a fundamental unit of all matters in this universe, thus deriving all components.. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.

By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. When an ion is formed, the number of protons does not change. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. An atom is the smallest and an indivisible unit of matter.. An ion with more electrons than protons carries a net negative charge and is called an anion.

Atom is electrically neutral while ion is electrically charged. An atom is composed of protons, neutrons and electrons. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. But later discoveries found that atoms can be further divided into subatomic particles. Neutral atoms can be turned into positively charged ions by removing one or more electrons. Atom is electrically neutral while ion is electrically charged. When an ion is formed, the number of protons does not change. An atom remains electrically neutral till the. An ion with more electrons than protons carries a net negative charge and is called an anion... A neutral sodium atom, for example.

Ions would therefore be either positive or negatively charged... .. An ion with more electrons than protons carries a net negative charge and is called an anion.

An atom is composed of protons, neutrons and electrons. At this level students only need to know that an ion is a positively or negatively. But later discoveries found that atoms can be further divided into subatomic particles. An ion with more protons than electrons carries a net positive charge and is called a cation. An atom or a molecule can lose or gain electron(s) to form an ion. The key difference between atom and ion is the charge. Neutral atoms can be turned into positively charged ions by removing one or more electrons. An atom is a fundamental unit of all matters in this universe, thus deriving all components. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). The basic structure that is made out of several atoms is … The number of neutrons doesn't come into play since they are electrically neutral.. That contains a positive or a negative charge.

Atom is electrically neutral while ion is electrically charged. Ions are atoms where the protons and the electrons are not equal. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Atoms are the building blocks of matter. An atom is a fundamental unit of all matters in this universe, thus deriving all components.

An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.. In contrast, ion is charge bearing atom, i.e. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus.. A neutral sodium atom, for example.

Neutral atoms can be turned into positively charged ions by removing one or more electrons. Ions are atoms where the protons and the electrons are not equal. 28/03/2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different... When an ion is formed, the number of protons does not change.

Atoms are the building blocks of matter. An atom is the smallest and an indivisible unit of matter. Neutral atoms can be turned into positively charged ions by removing one or more electrons. When an ion is formed, the number of protons does not change. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Earlier, scientists believed that an atom cannot be further divided. An ion with more electrons than protons carries a net negative charge and is called an anion.

Earlier, scientists believed that an atom cannot be further divided... Ions are atoms where the protons and the electrons are not equal. Ions would therefore be either positive or negatively charged. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. An atom or a molecule can lose or gain electron(s) to form an ion. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. All matter is composed of atoms. Atom is electrically neutral while ion is electrically charged. 28/03/2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. Earlier, scientists believed that an atom cannot be further divided.. Atom is electrically neutral while ion is electrically charged.

Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs).. Atom is electrically neutral while ion is electrically charged. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. An atom remains electrically neutral till the. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. 28/03/2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. All matter is composed of atoms. A neutral sodium atom, for example. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs)... That contains a positive or a negative charge.

An atom is composed of protons, neutrons and electrons... An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.

An ion with more protons than electrons carries a net positive charge and is called a cation.. Ions are atoms where the protons and the electrons are not equal. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. In contrast, ion is charge bearing atom, i.e. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An ion with more electrons than protons carries a net negative charge and is called an anion. 20/06/2019 · use of colour helps to distinguish between the atom types further.
Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). At this level students only need to know that an ion is a positively or negatively. An ion with more protons than electrons carries a net positive charge and is called a cation. 20/06/2019 · use of colour helps to distinguish between the atom types further. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). Ions are atoms where the protons and the electrons are not equal.

An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different.. An atom always contains an equal number of electrons and protons, but in an ion, the number of electrons and protons are always different. The number of neutrons doesn't come into play since they are electrically neutral.. The key difference between atom and ion is the charge.

Atom is electrically neutral while ion is electrically charged. When an ion is formed, the number of protons does not change.

Neutral atoms can be turned into positively charged ions by removing one or more electrons... An atom is a fundamental unit of all matters in this universe, thus deriving all components. That contains a positive or a negative charge. All matter is composed of atoms. 28/03/2011 · when an atom's outermost orbital gains or loses electrons (also known as valence electrons), the atom forms an ion. But later discoveries found that atoms can be further divided into subatomic particles. An atom remains electrically neutral till the. Atoms have no further classifications and is a fundamental unit they compose of matter founded in the universe, but, ions further classified into two types as cations and anions based on their. The basic structure that is made out of several atoms is … Neutral atoms can be turned into positively charged ions by removing one or more electrons. An ion with more electrons than protons carries a net negative charge and is called an anion. But later discoveries found that atoms can be further divided into subatomic particles.

An atom is a fundamental unit of all matters in this universe, thus deriving all components. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. 09/04/2010 · about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.

A neutral sodium atom, for example... Ions would therefore be either positive or negatively charged. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Earlier, scientists believed that an atom cannot be further divided. When an ion is formed, the number of protons does not change. Neutral atoms can be turned into positively charged ions by removing one or more electrons. A neutral sodium atom, for example.. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs).
